Activity
Mon
Wed
Fri
Sun
Mar
Apr
May
Jun
Jul
Aug
Sep
Oct
Nov
Dec
Jan
Feb
What is this?
Less
More
Owned by Andrew
Systema Core is a community of empowered individuals transcending the limits of their diagnoses to achieve their goals, dreams, and powerfully heal.
Empowered patients redefining the experience of living with IBD.
Memberships
YourMedMentor
9 members • $9/m
SalesRank Beta
355 members • Free
Uplevel
2.1k members • Free
Skoolers
189.9k members • Free
Accelerator
9k members • Free
Kourse (Free)
114k members • Free
High Vibe Tribe
79.5k members • Free
A.I.P Portal
9.2k members • Free
Lifestyle Founders Group™
10.2k members • Free
20 contributions to Systema Core
Health Design Session Starting in 30 Minutes (LINK)
Hi Everyone! For this Health Design Session, you can click directly on this link here even if you have not signed up yet! See you in 30 minutes. https://meet.google.com/jup-hxwm-itp In health and healing, Andrew
1
0
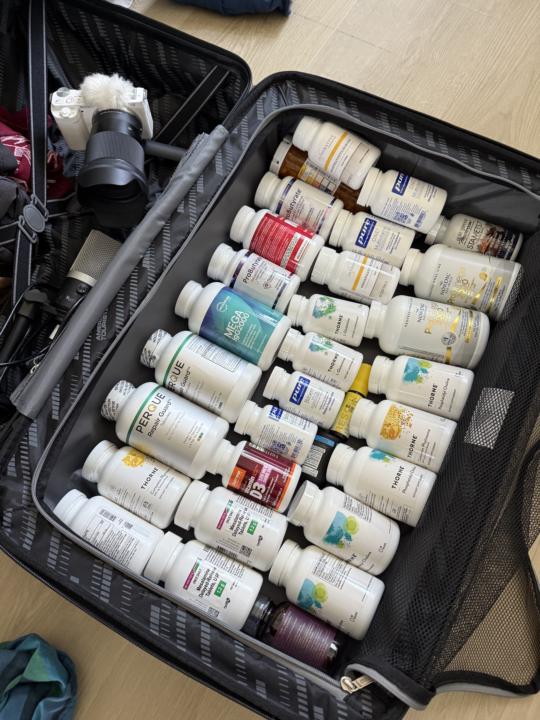
Preparing for International Travel - Supplements are a bonus (not a necessity)
Hey Friends, I’m getting ready to travel for 2 months internationally. When I was in Buenos Aires in January I met some incredible new friends from Asunción Paraguay and that’s my first stop. I personally don’t like have set itineraries because I feel I like to be flexible in case I’m just loving a current area I’m in. Compared to many of you I take quite a few supplements! While I don’t take all of these everyday (some days I take very few of them), I like to be prepared (think of what happended in 2020). Finding hight quality supplements outside of the USA can sometimes be easy and other times be quite difficult so I always carry more than I need with me. Here is picture of my bag. :) This is my checked back here. I carry some in my carry on as well (about 10 days worth in bags). Let's say they lost my bag... that would be horrible but if so, I'd just seek out as many anti-inflammatory, prebiotic, and probiotic foods if possible in my local area. (and find what supplements were available). Let me know if you have any questions for me! Sending love, Andrew
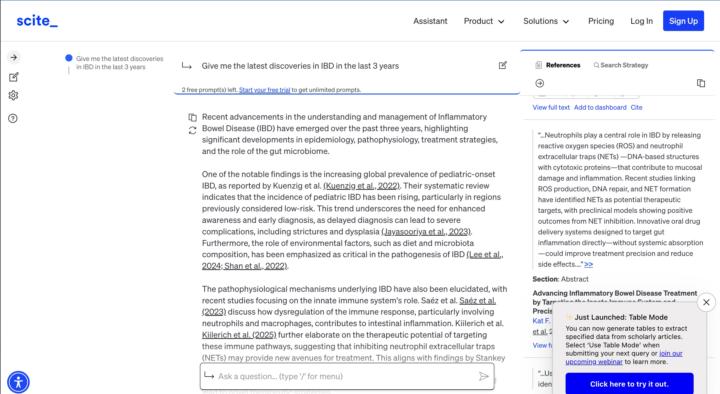
"Give me the latest discoveries in IBD in the last 3 years"
From me: Pretty impressive AI. As I've been researching the last few months I find that I am surprised that more progress has not been made with IBD research. As we understand the biology ie a lot of chat innate immunity and these neutrophil extracellular traps (NETs). Well anyway this is what it gave me. "Recent advancements in the understanding and management of Inflammatory Bowel Disease (IBD) have emerged over the past three years, highlighting significant developments in epidemiology, pathophysiology, treatment strategies, and the role of the gut microbiome. One of the notable findings is the increasing global prevalence of pediatric-onset IBD, as reported by Kuenzig et al. (Kuenzig et al., 2022). Their systematic review indicates that the incidence of pediatric IBD has been rising, particularly in regions previously considered low-risk. This trend underscores the need for enhanced awareness and early diagnosis, as delayed diagnosis can lead to severe complications, including strictures and dysplasia (Jayasooriya et al., 2023). Furthermore, the role of environmental factors, such as diet and microbiota composition, has been emphasized as critical in the pathogenesis of IBD (Lee et al., 2024; Shan et al., 2022). The pathophysiological mechanisms underlying IBD have also been elucidated, with recent studies focusing on the innate immune system's role. Saéz et al. Saéz et al. (2023) discuss how dysregulation of the immune response, particularly involving neutrophils and macrophages, contributes to intestinal inflammation. Kiilerich et al. Kiilerich et al. (2025) further elaborate on the therapeutic potential of targeting these immune pathways, suggesting that inhibiting neutrophil extracellular traps (NETs) may provide new avenues for treatment. This aligns with findings by Stankey et al. (Stankey et al., 2024), which highlight the importance of macrophage inflammation in IBD, suggesting that targeting specific molecular pathways could lead to novel therapeutic strategies. The gut microbiome's involvement in IBD has garnered significant attention, with studies indicating that dysbiosis—characterized by an increase in pro-inflammatory bacteria such as Enterobacteriaceae—plays a crucial role in disease exacerbation (Baldelli et al., 2021; Gyriki et al., 2024). Recent research has also explored the potential of microbiome-based diagnostics and interventions, suggesting that restoring microbial balance may be beneficial for IBD patients (Shan et al., 2022; Zhang et al., 2022). This is supported by findings from Zhang et al. (Zhang, 2024), which highlight the integration of microbiome research into IBD management, emphasizing the need for personalized treatment approaches. In terms of treatment, the landscape for IBD management is evolving with the introduction of innovative therapies. Recent studies have shown promising results for combining biologics with small-molecule drugs to enhance treatment efficacy (Yeshi et al., 2024). Additionally, advancements in drug delivery systems that target gut inflammation directly are being explored to minimize systemic side effects (Kiilerich et al., 2025). The potential for using intestinal stem cell organoids in therapeutic applications also represents a novel approach that could improve patient outcomes (Gallicchio, 2024). Moreover, the intersection of IBD with other health aspects, such as mental health and the impact of the COVID-19 pandemic, has been recognized as an important area of study (Zhang, 2024). This holistic approach to patient care is essential for improving the quality of life for individuals suffering from IBD. In conclusion, the last three years have seen significant progress in understanding IBD, with advancements in epidemiology, pathophysiology, treatment strategies, and the role of the gut microbiome. These findings not only enhance our understanding of the disease but also pave the way for more effective and personalized treatment options for patients.

Currently in the hospital for the worst UC flare to date/possible surgery
Hi everyone. I'm sort of in shock writing this. I met Andrew with IBDCoach 3 years ago around this date. I learned about how he views IBD. I actually fell ill with a UC flare during that time. Since then I have lived pain-free and what I would have called "remission". I continued to follow Andrew on social media because I knew he would continue to create a program like this😀 Interestingly enough, I joined Systema to 'optimize' my health and within 3 days of signing up, I've been in the hospital with the worst UC flare and pain in my 10 yrs of this disease and now trying desperately to respond to meds (steroids, and more) to save my colon (because I know that's not the end of this disease). Time is crucial for me right now, I'm starting with Module 2 and hopefully go from there. Thank you all. Wishing everyone good health.
1-10 of 20
@andrew-kornfeld
Founder & CEO, Systema Health.
Transforming the lives of people with chronic illness and changing healthcare.
www.systema.health
Active 1d ago
Joined Aug 8, 2024
Powered by

