Activity
Mon
Wed
Fri
Sun
Feb
Mar
Apr
May
Jun
Jul
Aug
Sep
Oct
Nov
Dec
Jan
What is this?
Less
More
Memberships
VS
Vagus School
500 members • Free
US
Ultra School
160 members • $7/m
3 contributions to Vagus School
Clinical Trial Alert: New Vagus Nerve Stimulation Study For Depression
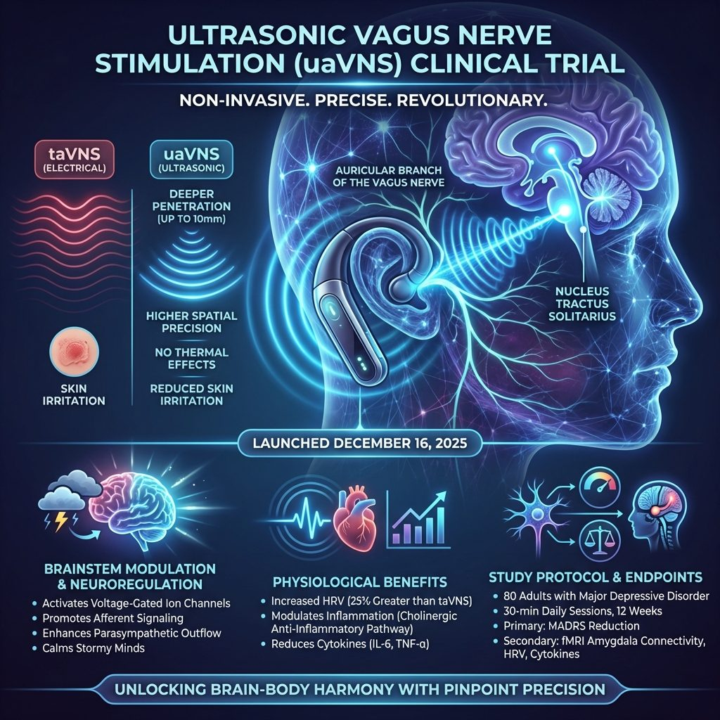
Clinical trial alert: Non-invasive ultrasonic auricular vagus nerve stimulation (uaVNS) is under investigation. Launched December 16, 2025, the trial employs low-intensity focused ultrasound (LIFU) via a wearable ear device. LIFU targets the auricular branch of the vagus nerve. Acoustic pressure waves mechanically activate voltage-gated ion channels. This promotes afferent signaling to brainstem nuclei, enhancing parasympathetic outflow. Inflammation is modulated via the cholinergic anti-inflammatory pathway. Compared to transcutaneous electrical VNS (taVNS), uaVNS offers superior penetration and spatial precision. No skin irritation. The double-blind, randomized, sham-controlled study includes 80 adults with major depressive disorder. Protocol: 30-min daily sessions for 12 weeks. Primary endpoint: MADRS reduction. Secondary: HRV, cytokine levels, fMRI. Preclinical rodent models show uaVNS yields a 25% greater HRV increase. Locus coeruleus norepinephrine modulation is 40% more robust than taVNS. https://clinicaltrials.gov/study/NCT07283913
USPro 2000 metal warming head
does anyone here have the small circle / dot appear on their 2000 head, if so is this a defective device or just use and age?
1 like • 2d
Interesting. I wonder what they will say about it. I've been wondering about my Ultrasound applicator head material. The manual says its aluminium. I've only had mine a week, not used the warming feature, and have only used a wet microfibre cloth to clean it. There are already some very fine scratches which leads me to think it is aluminium.
Switching from TENS based (like pulsetto) to Ultrasound
Is there anyone who has switched to Ultrasound by stopping use of TENS based solution? Doing both means we don’t know which one is working. :-)
1 like • 2d
I was using a Med-Fit 3 TENS for tVNS first with an ear clip and pad for ground, then the double ear clip electrode that goes either side tragus. It has been some time since I used the TENS, and I can say it was helpful for me, I just got out of the habit of doing it. I was using it a few times a week: approx 15 mins each time, 200μs PW, varying the frequency 15-30-20 Hz. I recently got a Med-Fit 1032 Ultrasound for PFC stimulation and discovered it could be used for vagus nerve, so I tried it out. I think it is better. I've been using it every day for 3-5 mins, and twice now it left me feeling calm with this expanded, grounded sense of awareness. Both have some downsides. The two TENS I own have analog control for intensity, and skin conductivity varied so I had to dial in intensity each time, being careful not to turn it up too high as that was so painful! Then there is that mild sound/sensation that was a bit annoying, along with my tragus sometimes feeling fatigued after use. The Ultrasound is quicker to use but as it's still new I'm very conscious of using it. I have long hair and the gel can get on it so I have to clean it off my neck/hair and applicator head afterwards. This device is larger and I think a little more fragile because of the ceramic transducer, so needs its padded case (which also holds gel and power adapter). I hear a tiny pulsing sound but I don't feel it, I only feel the coolness of the gel/applicator and I can move it slowly up/down my neck, which can be a little tiring because I am trying to position it carefully. If I had to choose which to take for a weekend, I would still bring the Ultrasound!
1-3 of 3




